Microglial Research: Key to Understanding Alzheimer’s Disease
Microglial research is revolutionizing our understanding of the brain’s immune system and its critical role in health and disease. These specialized cells, which assist in synaptic pruning and the maintenance of neural circuits, have emerged as key players in the pathology of neurodegenerative diseases, including Alzheimer’s disease. Discoveries led by prominent researchers like Beth Stevens highlight how dysfunction in microglial activity can lead to detrimental effects, furthering the progression of conditions such as Huntington’s disease. By uncovering the mechanisms of microglial behavior, researchers are paving the way for innovative therapies and biomarkers that could transform the landscape of treatment for millions suffering from Alzheimer’s. This transformative work exemplifies the importance of fundamental science in addressing complex health challenges that affect the brain.
Exploring the intricacies of glial cell function has become imperative in modern neuroscience, with a particular focus on these brain-residing immune entities. Known for their role in the brain’s defense and their involvement in processes like synaptic remodeling, these cells are crucial for maintaining cognitive health and function. Research by experts such as Beth Stevens underscores a shifting paradigm in how we view neuroinflammation and its implications for diseases characterized by neurodegeneration. The link between malfunctions in the brain’s immune response and disorders like Alzheimer’s disease has opened new avenues for therapeutic interventions and preventative strategies. As the scientific community delves deeper into this uncharted territory, the potential for groundbreaking advancements in treating neurological conditions only continues to grow.
Understanding Microglial Cells in Neurodegenerative Diseases
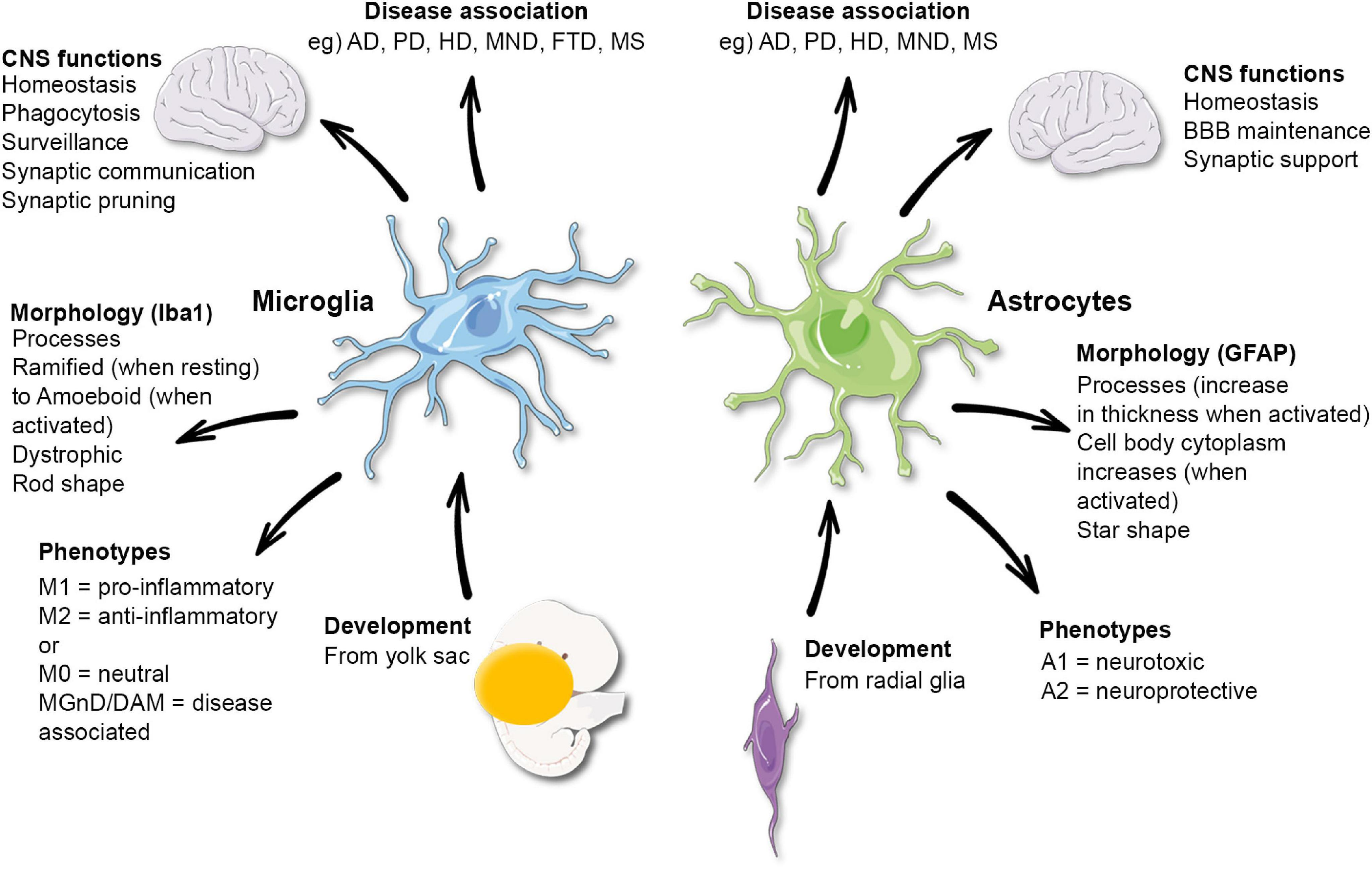
Microglial cells serve as the brain’s immune system, playing a crucial role in maintaining overall brain health. Their responsibilities include surveying the brain for signs of damage and swiftly removing dead or injured neurons. This process is vital for sustaining healthy communication between neurons, which is essential for proper brain function. However, the intricate balance of microglial activity is susceptible to disruption, which may contribute to neurodegenerative diseases such as Alzheimer’s. This highlights how crucial it is to understand the dual roles of microglia in both protecting and potentially harming the brain.
Recent research conducted by Beth Stevens at Boston Children’s Hospital has revealed that aberrant microglial cell activity, particularly in synaptic pruning, can have severe implications for neurodegenerative conditions. When microglia mistakenly prune healthy synapses alongside damaged ones, it can accelerate the progression of diseases such as Alzheimer’s. Recognizing these mechanisms is foundational for developing targeted therapies and diagnostic biomarkers that may one day help mitigate the impact of these debilitating conditions.
The Role of Synaptic Pruning in Alzheimer’s Disease
Synaptic pruning, a natural process carried out by microglial cells, is essential for the proper development and functioning of neural circuits. During early brain development, excessive synapses are trimmed down to optimize neural connectivity for effective communication between neurons. However, in the context of neurodegenerative diseases like Alzheimer’s, this process can become harmful. Incorrect or excessive synaptic pruning is hypothesized to disrupt the delicate balance of neural networks, potentially leading to cognitive deficits and memory loss.
Beth Stevens’ research into synaptic pruning has provided profound insights into this phenomenon. By studying the maladaptive pruning processes in mouse models, her lab has identified specific markers and pathways that could be targeted for therapeutic intervention in Alzheimer’s patients. This research not only deepens our understanding of how synaptic pruning influences neurodegeneration but also paves the way for innovations in treatment strategies aimed at preserving cognitive functions in patients suffering from Alzheimer’s disease.
Advancements in Alzheimer’s Research through Basic Science
The foundation of effective medical research lies in basic science, which often serves as the cornerstone for groundbreaking discoveries in Alzheimer’s treatment. Beth Stevens emphasizes the importance of curiosity-driven science in her work, revealing how essential studies can lead to unexpected breakthroughs in understanding complex neurodegenerative diseases. Her team’s exploration of microglial behavior has opened doors for investigating connections between brain immunity and Alzheimer’s disease, illustrating the trajectory from basic research to clinical application.
Through robust funding from federal agencies like the NIH, Stevens’ pioneering studies on microglia have contributed to a wealth of knowledge that could potentially transform future therapies for Alzheimer’s. As her lab delves deeper into these fundamental questions, the implications for patient care become increasingly significant. By elucidating the roles of microglial cells and their connection to neurodegenerative diseases, researchers are identifying pathways that may one day lead to innovative treatment strategies that address the underlying causes of Alzheimer’s, not just its symptoms.
The Impact of Federal Funding on Alzheimer’s Research
Federal funding plays a pivotal role in advancing research against Alzheimer’s disease and other neurodegenerative disorders. As highlighted by Beth Stevens, the essential support from agencies such as the National Institutes of Health allows scientists to pursue innovative ideas without the immediate pressure of clinical applicability. This long-term perspective fosters an environment in which novel hypotheses can be tested, leading to discoveries that may be indirectly related to the primary disease focus.
The sustained backing of federal bodies has resulted in significant strides in understanding microglial function and its link to Alzheimer’s pathology. By investing in foundational research, funding agencies enable researchers to take risks in unknown territories, ultimately leading to the identification of new biomarkers and therapeutic targets. Advocacy for continued investment in basic science can profoundly influence the landscape of neurological research, sparking further innovations that not only enrich scientific understanding but also enhance patient outcomes.
The Importance of Biomarkers in Alzheimer’s Disease
Biomarkers are critical in understanding Alzheimer’s disease progression and diagnosis. They provide measurable indicators that can help track the severity of the disease and response to treatment. Beth Stevens’ groundbreaking work on microglial function has illuminated potential biomarkers that are linked to neuroinflammation and synaptic pruning, offering new avenues for diagnosis and management of Alzheimer’s.
The development of biomarkers that reflect the activity of microglia in the brains of Alzheimer’s patients could revolutionize how the disease is diagnosed and monitored. By utilizing advanced imaging techniques and molecular profiling, researchers can identify specific changes that occur in the brain, allowing for earlier intervention and potentially delaying progression. As the field moves forward, the integration of these biomarkers into clinical practice will be paramount in transforming the landscape of Alzheimer’s care.
Exploring Synaptic Circuitry in Neurodegenerative Disease Research
Understanding the architecture of synaptic circuitry is crucial for comprehensive insights into neurodegenerative diseases like Alzheimer’s. Stevens’ research has focused on how microglial cells sculpt these circuits through synaptic pruning, emphasizing the delicate balance required for optimal cognitive function. Abnormalities in synaptic connections can lead to disrupted communication in the brain, which is a hallmark of Alzheimer’s pathology.
By utilizing animal models to explore synaptic circuitry dynamics, researchers can elucidate the changes that accompany neurodegeneration. These studies can reveal how alterations in microglial activity and synaptic pruning processes contribute to cognitive decline. This knowledge is vital for developing neuroprotective strategies that aim to preserve synaptic integrity, ultimately improving cognitive function in Alzheimer’s patients.
Neuroinflammation and Alzheimer’s Disease: A Complex Interaction
Neuroinflammation is increasingly recognized as a critical factor in the pathogenesis of Alzheimer’s disease. Microglial cells, while essential for immune defense, can become overactivated in response to pathological changes in the brain. This persistent inflammation can exacerbate neuronal damage and contribute to cognitive decline. Stevens’ research emphasizes the dual role of microglia in neuroinflammatory responses and their impact on Alzheimer’s disease progression.
Understanding the mechanisms behind neuroinflammation is crucial for developing targeted therapies that can modulate microglial activity. By exploring the signaling pathways involved in neuroinflammatory processes, researchers can devise strategies aimed at repairing neuronal damage and slowing disease progression. Addressing neuroinflammation represents a promising avenue for novel treatment approaches that may significantly improve outcomes for individuals living with Alzheimer’s.
Implications of Neurodegenerative Disease Research for Patient Care
The research advancements in understanding microglial function and synaptic pruning hold significant implications for patient care in Alzheimer’s disease. As scientists, including Beth Stevens, uncover the intricate details of how neuroinflammation and microglial activity relate to cognitive decline, these insights can inform clinical practices. Physicians armed with this knowledge can better tailor interventions and support for patients, possibly enhancing their quality of life.
Moreover, the quest for effective treatments grounded in this research extends hope to the millions impacted by Alzheimer’s. The identification of new biomarkers and therapeutic targets derived from comprehensive studies on neurodegenerative diseases not only aids in early diagnosis but also helps in developing drugs that target the underlying mechanisms of the disease. Ultimately, these efforts can lead to breakthroughs in Alzheimer’s treatment, fostering a proactive approach to managing this challenging condition.
Future Directions in Microglial Research and Alzheimer’s Disease
The future of Alzheimer’s research is deeply intertwined with advancements in understanding the role of microglial cells in neurodegeneration. As scientists delve deeper into the complexities of how microglia interact with neurons and respond to damage, new therapeutic strategies are likely to emerge. Innovations in technology, such as single-cell sequencing and advanced imaging, will continue to enhance our understanding of microglial behavior in the context of Alzheimer’s.
Additionally, interdisciplinary collaborations will be essential in driving forward this research landscape. By integrating insights from molecular biology, neurology, and clinical practice, researchers can holistically approach Alzheimer’s disease treatment. The ultimate goal is to translate these discoveries into clinical applications that improve patient outcomes and offer hope to those affected by neurodegenerative diseases.
Frequently Asked Questions
What role do microglial cells play in Alzheimer’s disease research?
Microglial cells are crucial in Alzheimer’s disease research as they represent the brain’s immune system. They patrol the brain to detect illness and injury, clear out dead cells, and prune synapses. Aberrant microglial pruning can lead to neurodegenerative diseases like Alzheimer’s, making them a key focus for developing new treatments and biomarkers.
How do microglia contribute to synaptic pruning in neurodegenerative diseases?
Microglia contribute to synaptic pruning by selectively removing unnecessary synapses, which is vital for proper brain function. However, in neurodegenerative diseases like Alzheimer’s and Huntington’s, this process can become dysfunctional, leading to excessive synapse loss and contributing to cognitive decline.
Who is Beth Stevens and what is her contribution to microglial research?
Beth Stevens is an influential neuroscientist known for her pioneering work on microglial cells and their role in neurodegenerative diseases. Her research has significantly advanced our understanding of how microglia affect synaptic pruning and immune responses in the brain, paving the way for new treatments for conditions like Alzheimer’s disease.
What discoveries have been made about microglia’s functions in the healthy brain?
Research has shown that microglia perform essential functions in a healthy brain, including maintenance of synapse integrity and responding to cellular damage. By engaging in synaptic pruning during development, they help sculpt neuronal circuits that are crucial for learning and memory.
How does microglial dysfunction relate to neurodegenerative diseases?
Microglial dysfunction can lead to inadequate response to neuronal injury and excessive synaptic pruning, contributing to neurodegenerative diseases such as Alzheimer’s. Understanding these mechanisms is critical in developing targeted therapies that restore microglial function and protect neuronal health.
What impact does microglial research have on potential treatments for Alzheimer’s?
Microglial research significantly impacts potential Alzheimer’s treatments by identifying new biomarkers and therapeutic targets. By understanding how microglia contribute to neuroinflammation and neurodegeneration, researchers can develop strategies to modify their activity, potentially slowing disease progression and improving patient outcomes.
What are the future directions of microglial research in neurodegenerative diseases?
Future directions of microglial research include exploring their roles in synaptic health, modulation of inflammation, and their interactions with other brain cells. This research aims to uncover novel therapeutic possibilities for neurodegenerative diseases like Alzheimer’s, focusing on restoring normal microglial function.
How does federal funding support microglial research in Alzheimer’s disease?
Federal funding, primarily from the NIH, has been vital for advancing microglial research in Alzheimer’s disease. It enables scientists like Beth Stevens to pursue curiosity-driven projects that can lead to groundbreaking discoveries about the brain’s immune system and its implications for treating neurodegenerative disorders.
| Key Point | Details |
|---|---|
| Microglial Cells | Serve as the immune system for the brain, clearing out dead cells and pruning synapses. |
| Role in Neurodegenerative Diseases | Aberrant pruning by microglia is linked to Alzheimer’s, Huntington’s, and other disorders. |
| Research Foundation | Funded primarily by NIH and federal agencies, allowing exploration of critical questions in neuroscience. |
| Impact on Treatment | Research on microglia may lead to new biomarkers and medicines for Alzheimer’s and similar diseases. |
| Curiosity-Driven Science | Basic science studies are crucial for uncovering new understandings that can translate to treatments. |
Summary
Microglial research is reshaping our understanding of brain health and disease. It highlights the role of microglial cells as critical immune defenders in the brain while also revealing their potential detrimental effects in neurodegenerative conditions. The research led by scientists like Beth Stevens not only sheds light on the mechanisms underlying diseases such as Alzheimer’s but also opens pathways toward innovative treatments and biomarkers that could improve the quality of life for millions affected by these disorders.